
Cystatin C provides a more accurate estimation of GFR to confirm CKD & monitor kidney function
Cystatin C is more stable and applicable in diverse groups
Not affected by external factors that affect serum creatinine such as, dietary intake (including high protein diets), muscle mass, and secretion by the kidney.
Cystatin C is more accurate
At higher levels of eGFR, in particular, for patients with stage 3 CKD, serum creatine underestimates true renal function, sometimes resulting in over-diagnosis of CKD.
For the following clinical scenarios, Cystatin C eGFR is the preferred test.
Kidney function for individuals classified as black or mixed-race, as creatinine-based eGFR may be systematically biased.
Kidney function for individuals with extremes of muscle mass or dietary protein, as unlike serum creatinine, Cystatin C eGFR is unaffected by diet or muscle mass e.g., muscular athletes, those on high protein diets, or in frail older adults.
Individuals with creatinine-based eGFR of 45-59 ml/min/1.73m² in the absence of elevated urine albumin; if Cystatin C eGFR is > 60 ml/min/1.73m² in these cases, the individual does not have CKD.

Cystatin C as a confirmatory test for CKD
- No albuminuria,
- A serum creatinine eGFR of 45 – 59 ml/min/1.73m²
- A Cystatin C eGFR > 60 ml/min/1.73m²
Cystatin C has been recognized for use by:
The American Kidney Fund (AKF): Stated that Cystatin C, when used alongside serum creatinine and albuminuria testing, provides the most informative CKD diagnosis and risk stratification.
Kidney Disease Improving Global Outcomes (KDIGO): Concluded that Cystatin C is a critical component of accurate risk stratification as Cystatin C strengthens the associations of eGFR and cardiovascular events, kidney failure, and death.
The National Institute for Health and Care Excellence (NICE): acknowledged the use of Cystatin C-based testing to estimate eGFR.
Alpha Labs is evaluating the potential for the use of Cystatin C eGFR test in Ontario’s Primary Care setting
Alpha Labs together with national and international experts is evaluating the use of Cystatin C eGFR for improved effectiveness and efficiency in the diagnosis and treatment of chronic kidney disease in Ontario.
We need Primary Care Partners to help us achieve our goal of providing the right tools to support patient care.
Join the Alpha Labs Best Tests™ Community as a Primary Care Partner to evaluate the potential of Cystatin C as an OHIP SOB-LS listed test.
How can you contribute?





Additional information about Cystantin C eGFR to help your patients
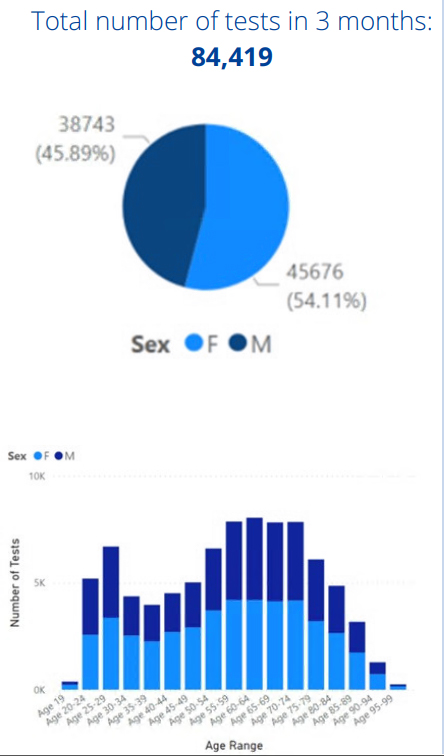
Preliminary Alpha Labs pilot study data
- 16% of all tests had creatinine eGFR < 60 ml/min/1.73m² (13,121 / 84,419)
- 9% of all tests had creatinine eGFR 45 – 59 ml/min/1.73m² (7,345 / 84, 419)
- Of all those with creatinine eGFR 45 – 59 ml/min/1.73m², 20% (1,464 / 7,345) had Cystatin C eGFR > 60 ml/min/1.73m² and can be classified as not having CKD
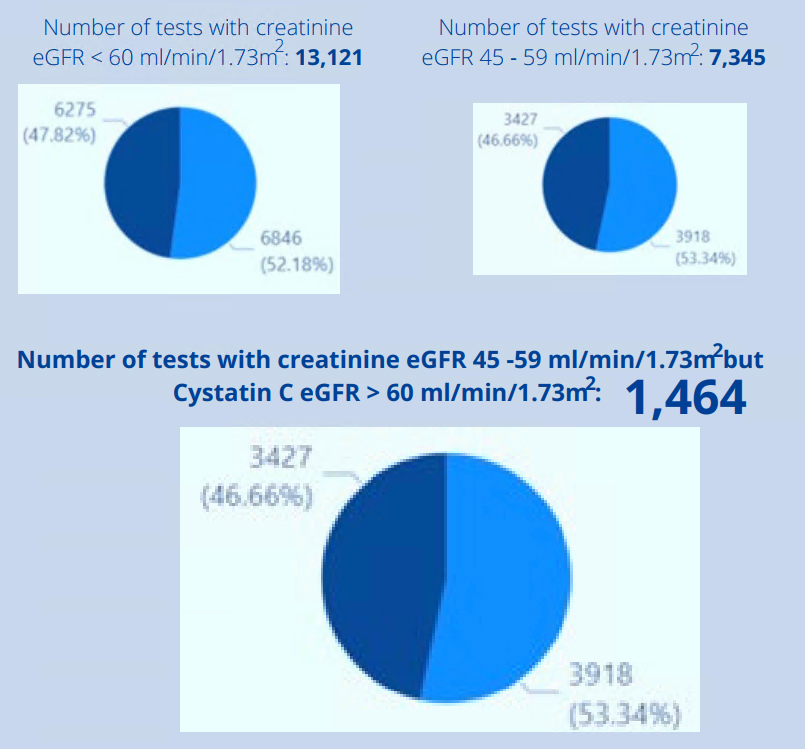
This means that several patients were informed they had an abnormal result when in fact they do not.
As per international guidelines and the CCO-Ontario Renal Network KidneyWise Clinical Toolkit for primary care (www.kidneywise.ca), primary care providers should repeat testing for those patients with initial abnormal results (creatinine below 60 ml/min/1.73m²) three (3) months later (or as soon as possible thereafter) to confirm a diagnosis of CKD.
If follow-up creatinine eGFR results remain abnormal (<60) while the Cystatin C eGFR remains with the normal range (>60), patients are at risk of being misdiagnosed with CKD. This will result in unnecessary additional tests and medications, other costs to publicly funded health care, and implications for the access to or cost of private health insurance plans. Most importantly, patients will appreciate the reassurance of not being labeled inaccurately with a chronic disease.
Until the end of the project (November 2021), patients 50 years and older referred to Alpha Labs for a creatinine eGFR test (CKD-EPI Creatinine equation) will also have a Cystatin C eGFR result (CKD-EPI Cystatin C equation) reported along with it.
Project Leads






Alpha Labs’ Best Tests™ Ontario
Family physicians and nurse practitioners in Ontario deserve the best tools possible to help detect, monitor and manage their patients’ conditions
Alpha Labs Best Test OntarioTM is working with national and international experts in community practice to carry out innovative projects to create the best tools and make them available to primary care clinicians. Projects are funded in part by the Ministry of Health’s New Tests and Technologies Fund (NTTF).